When it comes to understanding the fundamental concepts of physics, one can’t overlook the importance of electrons. These subatomic particles play a pivotal role in determining the charge of an object. When an object gains extra electrons, it becomes negatively charged – a fascinating phenomenon that’s often overlooked.
This article delves into the intriguing world of electrons, specifically focusing on how an excess can alter an object’s charge. It’s a journey into the heart of atomic structures, shedding light on how the world around us operates at a microscopic level.
Whether you’re a science enthusiast or a curious reader, this exploration of electrons promises to be an enlightening experience.

Benda Yang Kelebihan Elektron Akan Bermuatan
Benda yang kelebihan elektron akan bermuatan is a phrase in Bahasa Indonesia, meaning an object with excess electrons will bear a charge. This section delves into the concept of excessive electrons leading to a net charge. Maintain the theme of electron importance, originating from the earlier part of the article, while introducing new layers to the topic.
What is “benda yang kelebihan elektron akan bermuatan”?

A balance exists between the number of electrons and protons under standard conditions, upholding the neutrality of the object.
However, when the object acquires more electrons than protons, it becomes negatively charged. It’s due to the fact that electrons carry a negative electric charge. As a result, an object with increased electrons carries a surplus of negative charge, solidifying its status as benda yang kelebihan elektron akan bermuatan.
Elemental Basics and Electrons
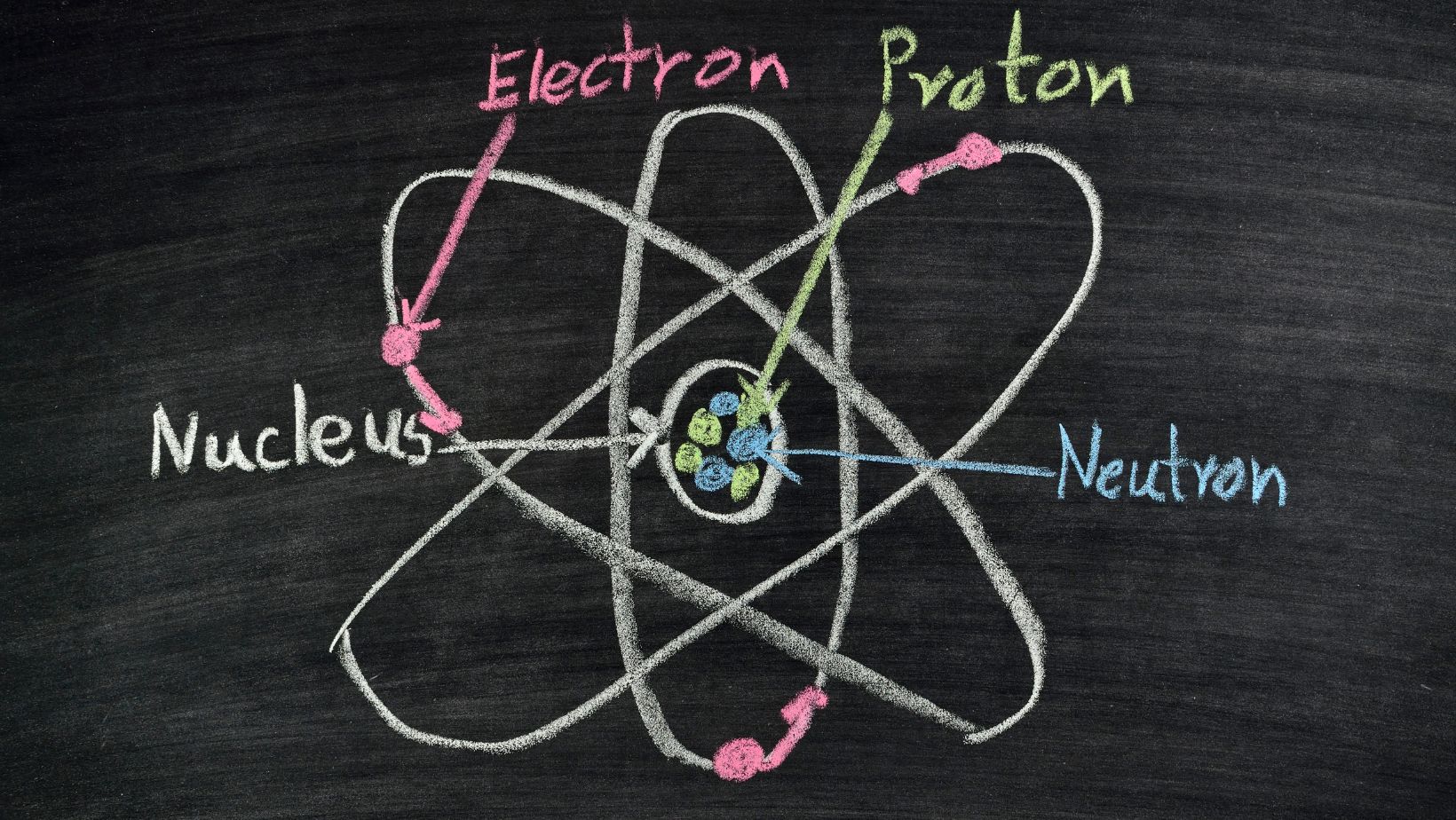
Understanding the role electrons play in charge dynamics begins with grasping the elemental basics. Each element consists of atoms, the building blocks of matter. At the heart of an atom, there’s a nucleus housing protons with positive charges and neutrons with no charge. Circling this nucleus, electrons inhabit energy levels.
Normally, an atom remains neutral as the number of electrons equals the protons’ count. However, through interactions like friction or induction, atoms might gain or lose electrons. Gaining electrons, the atom becomes negatively charged, thus the formation of benda yang kelebihan elektron akan bermuatan. On the contrary, losing electrons leaves the atom positively charged.

It’s a simple but fundamental principle shaping the interaction of matter at micro and macro levels. Integral to physics, this principle remains a vibrant field of study, continuously revealing intricate complexities of the universe’s tapestry.
Impact of Excess Electrons on Objects
Objects laden with excess electrons undergo notable transformations. These changes primarily reflect on two facets – physical properties and chemical behavior.
Alteration of Physical Properties
Objects possessing excess electrons experience changes in their physical properties. When an excess of electrons inhabit their atomic orbitals, they become negatively charged(source). Observably, these objects attract positively charged elements, a clear manifestation of electrostatic forces in action. For instance, a negatively charged plastic comb attracts tiny paper bits. This phenomenon unfolds due to the excess electrons in the comb generating an attractive force towards the positively charged or neutral paper bits.

Changes in Chemical Behavior
Besides physical alteration, excess electrons also induce changes in an object’s chemical behavior. Electrons are key players in chemical bonding. Their surfeit in an object can affect reactions and formation of bonds, significantly altering the chemical nature of entities involved.
For example, in ionic bonding, atoms with electron surfeit become anions (negatively charged ions) by gaining electrons, thereby promoting them to partake in bond formation with cations (positively charged ions). Similarly, in redox reactions, such entities serve as reducing agents, giving up their excess electrons.

This change influences chlorine’s ability to react with other substances, essentially modifying its chemical reactivity. Thus, excess electrons inevitably lead to new, and sometimes unexpected, chemical behaviors.
Practical Examples of benda yang kelebihan elektron akan bermuatan
Manifestations of objects with excess electrons appear frequently in daily life and nature. For instance, static electricity results from the transfer of excess electrons. When rubbing a balloon against hair, electrons move from the hair to the balloon, rendering the balloon negatively charged. The charged balloon attracts positively charged items, such as tiny pieces of paper due to the electrostatic force.
Another compelling representative of objects with surplus electrons is lightning during a thunderstorm. Cloud atoms capture extra electrons, creating an intense negative charge within the thundercloud. As a contradiction, Earth’s surface develops a strong positive charge, leading to a build-up of electrostatic force. Eventually, the electrostatic force discharges as a bolt of lightning, a powerful demonstration of objects with excess electrons.

Benda Yang Kelebihan Elektron Akan Bermuatan – Electrons in Action
The presence of excess electrons is indeed a game-changer. It’s this surplus that gives an object its charge, leading to fascinating phenomena and technological advancements. From the static electricity that makes your hair stand on end to the brilliant flash of a lightning bolt, it’s all about the electron surplus. Even the functionality of our everyday electronic devices and the formation of common substances like table salt owe it to these extra electrons. So, it’s not just about atoms gaining or losing electrons. It’s about how these excess electrons shape our world, influencing both natural phenomena and human-made technologies.
Bob Duncan is the lead writer and partner on ConversationsWithBianca.com. A passionate parent, he’s always excited to dive into the conversation about anything from parenting, food & drink, travel, to gifts & more!
